STUDY ON THE EFFECT OF TEMPERATURE ON THE CALCINATION TREATMENT TECHNIQUE FOR OBTAINING SILICOPHYTOLITH
DOI:
https://doi.org/10.5710/PEAPA.01.08.2023.470Keywords:
Phytoliths, Morphometry, Arundo donax L, Bilobate, CalcinationAbstract
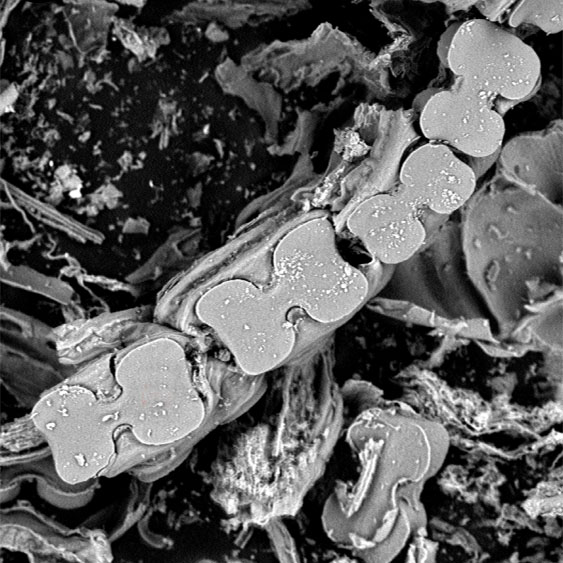
This paper presents the analysis of the variation in size and shape of BILOBATE silicophytolith in leaves of Arundo donax L. processed by calcination at different temperature ranges (specifically at 500 ºC, 650 ºC, 800 ºC, and 950 ºC). The morphometric variables used were size and shape (e.g., length, width, perimeter, area, convex perimeter, convex area, shape factor, roundness, convexity, solidity, and aspect ratio). The phytolith association for this species was characterized by the presence of ELONGATE morphotypes, BULLIFORM FLABELLATE, BILOBATE, POLYLOBATE, CROSS, and stomatal structures, both unicellular and multicellular. The phytolith variability of leaves processed at 950 ºC showed a lower number of CROSS and BILOBATE and a greater abundance of ELONGATE and BULLIFORM FLABELLATE silicophytoliths. Meanwhile, the number of articulated BILOBATE phytoliths decreased and the articulated BULLIFORM FLABELLATE phytoliths increased. The BILOBATE morphotypes were selected for morphometric analysis because of their high frequency. When processed at 950 ºC, they show color and shape variations, indicated by high values of convexity, solidity, shape factor and roundness, and size (represented by perimeter reduction). These results would indicate a trend in the change in size, expressed as a reduction in the perimeter, and a variation in shape, related to greater sphericity and convexity of the morphotypes. Complementary observations were made by SEM and SEM-EDS analysis, showing silica loss in silicophytoliths processed at 950 ºC. The present work contributes to the methodological knowledge of processing plant material for silicophytolith extraction.
References
Abramoff, M. D., Magelhaes, P. J. y Ram, S. J. (2004). Image Processing with ImageJ. Biophotonics International, 11, 36–42.
Arana, M. D., Natale, E., Ferretti, N., Romano, G., Oggero, A., Martínez, G., Posadas, P., Morrone, J. J. (2021). Esquema biogeográfico de la República Argentina. Opera lilloana 56, Fundación Miguel Lillo, Tucumán, Argentina.
Ball, T. (1996). Identifying Phytoliths Produced by the Inflorescence Bracts of Three Species of Wheat (Triticum monococcum L., T. dicoccon Schrank., and T. aestivum L.) Using Computer-Assisted Image and Statistical Analyses. Journal of Archaeological Science, 23, 619–632.
Ball, T. B. y Brotherson, J. D. (1992). The Effect of Varying Environmental Conditions on Phytolith Morphometries in Two Species of Grass (Bouteloua curtipendula and Panicum virgatum), Scanning Microscopy: Vol. 6: No. 4, Article 27.
Ball, T. B., Brotherson, J. D. y Gardner, J. S. (1993). A typologic and morphometric study of variation in phytoliths from einkhorn wheat (Triticum monococcum L.). Canadian Journal of Botany, 71, 1182–1192.
Ball, T. B., Ehlers, R. y Standing, M. D. (2009). Review of typologic and morphometric analysis of phytoliths produced by wheat and barley. Breeding Science, 59, 505–512.
Ball, T., Davis A. L., Evett, R., Ladwig, K., Tromp, M., Out, W., Portillo, M. (2016). Morphometric analysis of phytoliths: recommendations towards standardization from the International Committee for Phytolith Morphometrics. Journal of Archaeological Science, 68, 106–111.
Ball, T., Vrydaghs, L., Mercer, T., Pearce, M., Snyder, S., Lisztes-Szabó, Z., Peto, Á. (2017). A morphometric study of variance in articulated dendritic phytolith wave lobes within selected species of Triticeae and Aveneae. Vegetation History and Archaeobotany, 26, 85–97.
Bertoldi de Pomar, H. (1971). Ensayo de clasificación morfológica de los silicofitolitos. Ameghiniana, 8, 317–328.
Bowdery, D. (1989). Phytolith analysis: Introduction and applications. In Beck, W., Clarke, A., Head, L (Eds.), Plants in Australian archaeology: Archaeology and material culture studies in anthropology (Vol. 1, pp. 161–186). Brisbane: Watson Ferguson & Company.
Boyd, M. (2002). Identification of anthropogenic burning in the paleoecological record of the Northern Prairies: a new approach. Annals of the Association of American Geographers 92, 471–487.
Cabrera, A.L. (1971). Fitogeografia de la República Argentina. Boletín de la Sociedad Argentina de botánica 14, 1–50.
Cabrera, A. L. y Willink, A. (1973). Biogeografía de América Latina: Monografía 13, Serie de Biología. 123 pp.
Chauhan., D. K., Tripathi, D. K., Kumar, D., Kumar., Y. (2011). Diversity, Distribution and Frequency Based Attributes of Phytolith in Arundo donax L. International Journal of Innovations in Biological and Chemical Sciences, 1, 22–27.
Cooke, J., Degabriel, J. L. y Hartley, S. E. (2016). The functional ecology of plant silicon: geoscience to genes. Functional Ecology. 30, 1270–1276.
Cravero, S. A. C., Bianchi, C. L., Elena, H. J., Bianchi, A. R. (2017). Clima de la Argentina Mapas digitales mensuales de precipitaciones y precipitación menos evapotranspiración potencial: adenda del Atlas Climático digital de la República Argentina. Ed. -Salta: Ediciones INTA.
Dinerstein, E., Olson, M., Graham, D. J., Webster, A. L., Primm, S. A., Bookbinder, G., Ledec, M. P. (1995). Una Evaluación del Estado de Conservación de las Ecorregiones de América Latina y el Caribe. Publicación. Banco Mundial – Fondo Mundial para la Naturaleza, 13500, Washington, 136 pp.
Elbaum, R. y Weiner, S. (2003). Detection of Burning of Plant Materials in the Archaeological Record by Changes in the Refractive Indices of Siliceous Phytoliths. Journal of Archaeological Science, 30, 217–226.
Ellis, R. P. (1979). A procedure for standardizing comparative leaf anatomy in the Poaceae. II. The epidermis as seen in surface view. Bothalia 12(4), 641–671.
Epstein, E. (2009). Silicon: its manifold roles in plants. Annals of applied Biology, 155, 155–160.
Hammer, Ø., Harper, D. A. T., Ryan, P. D. (2007). PAST – Palaeontological STatistics, 1.75. 86pp.
Head, H. H. (1962). Rutley’s Elements of Mineralogy. London: Thomas Murby & Co., Guildford, The Woodbridge Press, Ltd, pp. 152–155.
Hitchcock, A. S. (1951). Man. Grasses U.S. (ed. 2) 1–1051. U.S. Department of Agriculture, Washington, D.C.
Jones, R. L. y Beavers, A. H. (1963). Some mineralogical and chemical properties of plant opal. Soil Sciencie, 96, 375–379.
Jones, L. H. P. y Milne, A. A. (1963). Studies of silica in the oat plant. 1. Chemical and physical properties of the silica. Plant and Soil 18(2), 207–220.
Jones, J. B. y Segnit, E. R. (1969). Water in sphere-type opal. Mineralogical magazine, 37(287), 357–361.
Katz, O. (2019). Conflict and complementarity of paleontological and molecular chronologies. Paleobiology 45(1), 7–20.
Labouriau, L. G. (1983). Phytolith work in Brazil, A minireview. The phytolitarien Newsletter, 2(2), 6–11.
Lanning, F. C., Ponnaiya B. W. X. y Crumpton, C. F. (1958). The Chemical Nature of Silica in Plants. Plant Physiol, 33, 339–343.
Lu, H., Zhang, J., Wu, N., Liu, K-b, Xu, D., Li, Q. (2009). Phytolith Analysis for the Discrimination of Foxtail Millet (Setaria italica) and Common Millet (Panicum miliaceum). PLOS Global Public Health, 4, 4448.
Martínez Carretero, E., Faggi, A. M., Fontana, J. L., Aceñolaza, P., Gandullo, R., Cabido, M., Iriart, D., Prado, D., Roig, F.A. y Eskuche, U. (2016). Prodromus Sinsistemático de la República Argentina y una Breve Introducción a los Estudios Fitosociológicos. Boletín de la Sociedad Argententina de Botánica, 51(3), 469–549.
Neumann, K., Strömberg, C. A. E., Ball, T., Albert, R. M., Vrydagh, L., Scott Cumming, L. (2019). International code for phytolith nomenclature (ICPN) 2.0. Annals of Botany. Oxford, 20, 1–11.
Out, W. A. y Madella, M. (2015). Towards improved detection and identification of crop by-products: morphometric analysis of bilobate leaf phytoliths of Pennisetum glaucum and Sorghum bicolor. Quaternary International, 434, 1–14.
Out, W. y Madella, M. (2016). Morphometric distinction between bilobate phytoliths from Panicum miliaceum and Setaria italica leaves. Archaeological and Anthropological Sciences, 8(3), 505–521.
Oyarzabal, M., Clavijo, J., Oakley, L., Biganzoli, F., Tognetti, P., Barberis, I., Maturo, H. M., Aragón, R., Campanello, P. I., Prado, D., Oesterheld, M., y León, R. J. C. (2018). Unidades de vegetación de la Argentina. Ecología Austral 28, 040–063.
Parr, J. F. (2006). Effect of fire on phytolith coloration. Geoarchaeology: An International Journal, 21(2), 171–185.
Parr, J. F., Lentfer, C. J. y Boyd, W. E. (2001). A comparative analysis of wet and dry ashing techniques for the extraction of phytoliths from plant material. Journal of Archaeological Science, 28, 875–886.
Patterer, N. I., Passeggi, E. y Zucol, A. F. (2011). Análisis fitolíticos de suelos del sudoeste de la Provincia de Entre Ríos (Argentina) como una herramienta para comprender sus procesos pedológicos. Revista Mexicana De Ciencias Geológicas, 28(1), 132–146.
Payá, J., Roselló, J., Monzó, J. M., Escalera, A., Santamarina, M. P., Borrachero, M. V.., Soriano, L. (2018). An Approach to a New Supplementary Cementing Material: Arundo donax Straw Ash. Sustainability, 10, 4273.
Pearsall, D. M. (1989). Paleoethnobotany: a Handbook of Procedures. Academic Press.
Piperno, D. R. (2006). Phytoliths: A Comprehensive Guide for Archaeologists and Palaeoecologists. Alta Mira Press, Lanham, p. 238.
Rovner, I. (1971). Potential of opal phytolith for use in paleoecological reconstruction. Quaternary Research, 1, 343–359.
Rovner, I. (1972). Note on a safer procedure for opal phytolith extraction. Quaternary Research, 2(4), 591.
Runge, F. (1998). The effect of dry oxidation temperatures (500–800°C) and of natural corrosion on opal phytoliths. In J.D. Meunier, F. Colin, & L. Faure-Denard (Eds.), The phytoliths: Applications in earth science and human history (p. 73). Aix en Provence, France: CEREGE. San Diego, California.
Terruggi, M. E. (1955). Algunas observaciones microscópicas sobre vidrio volcánico y ópalo organógeno en sediemntos pampioanos. Notas del museo de La Plata 18, Geología 66, 17–26
Wallis, L. (2003). An overview of leaf phytolith production patterns in selected northwest Australian flora. Review of Palaeobotany & Palynology, 125, 201–248.
Wu, Y., Yang, Y., Wang, H. (2014). The effects of chemical composition and distribution on the preservation of phytolith morphology. Applied Physics A, 114, 503–507.
Zucol, A. F. (1996). Microfitolitos de las Poaceae argentinas: I. Microfitolitos foliares de algunas especies del género Stipa (Stipeae: Arundinoideae), de la Provincia de Entre Ríos. Darwiniana, 34, 151–172.
Zucol, A. F., Brea, M., Passeggi, E. y Franco, M. J. (2014). Colecciones del laboratorio de paleobotánica y procesamiento de material sedimentario del CICYTTP- Diamante (CONICET), Entre Ríos, Argentina. Boletín de la Asociación Latinoamericana de Paleobotánica y Palinología, 14, 71–82.
Zucol, A.F. y Osterrieth, M. (2002). Técnicas de preparación demuestras sedimentarias para la extracción de fitolitos. Ameghiniana, 39, 379–382.
Additional Files
Published
Issue
Section
License
Copyright (c) 2023 Sebastián Ariel Frezzia, Noelia Isabel Patterer, Alejandro Fabián Zucol, Esteban Passeggi

This work is licensed under a Creative Commons Attribution-NoDerivatives 4.0 International License.
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Atribución/Reconocimiento 4.0 Internacional that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.















